Write a Mechanism of the Following Reaction:
The reaction is as follows. The rate-determining step of this reaction depends on the interaction between the two species namely the nucleophile and the organic compound.

The Mechanism Of Base Catalyzed Hydrolysis Of Nitriles Organic Chemistry Chemistry Lessons Organic Chemistry Study
I appreciate your attention to Write A Mechanism For The Following Radical Halogenation Reaction detail and promptness.
. KCN K CN- n Butylbromide n- butylcyanide. Proptonation of ethene to form carbocation by electrophilic attack of H 3 O. The given reaction is.
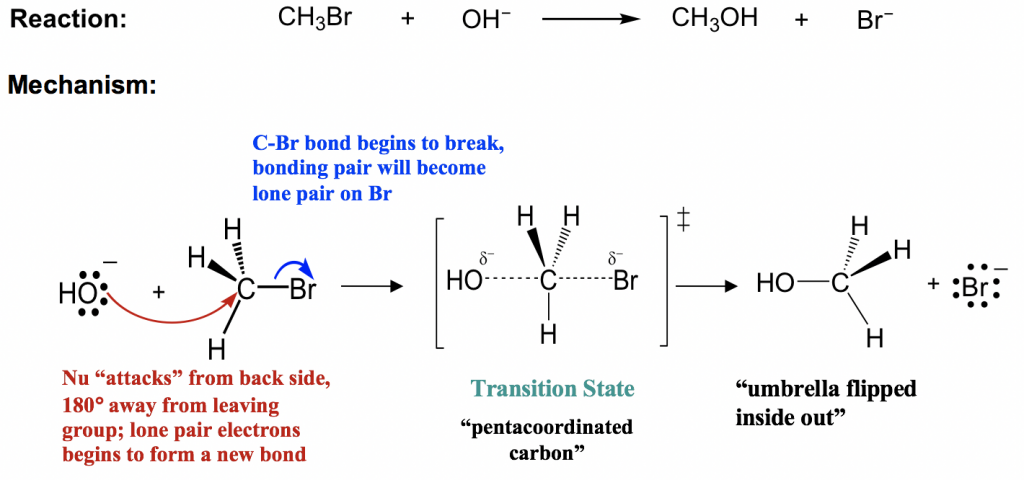
The given reaction is an S N 2 reaction. In this reaction CN - acts as the nucleophile and attacks the carbon atom to which Br is attached. Deprotonation of alcohol ii Mechanism of dehydration of ethanol to ethene.
Formation of Protonated alcohol. 2 C H 3 C H 2 OH H. Write a mechanism of the following reaction.
Write the mechanism of the following reaction. The given reaction follows S N 2 mechanism as shown below. CBSE Class 12 Chemistry.
Alkanes - Reactions of Haloalkanes - Nucleophilic. A Write the mechanism of the following reaction. Formation of protonated alcohol.
Cyanide ion acts as a nucleophile and bromide ion acts as leaving group. The S N 2 reaction mechanism involves the nucleophilic substitution reaction of the leaving group which generally consists of halide groups or other electron-withdrawing groups with a nucleophile in a given organic compound. Formation of carbocation slow step Step 3.
2 C H 3 C H 2 O H H C H 3 C H 2 O C H 2 C H 3 b Write the equation involved in the acetylation of salicyclic acid. 2CH 3 -CH 2 -OH -- CH 3 -CH 2 -O-CH 2 -CH 3. 5 Write a mechanism that explains the following reaction.
CH_3CH_2OH oversetHBr toCH_3CH_2Br H_2O b Write the equation involved in Reimer-Tiemann reaction. The overall reaction rate depends almost entirely on the rate of the slowest step. NBuBrKCN EtOHH 2 O nBuCN Medium Solution Verified by Toppr This is an example of bimolecular nucleophilic substitution reaction S N 2.
Write the mechanism of the following reaction. In this reaction CN acts as the nucleophile and attacks the carbon atom to which Br is attached. To Keep Reading This Answer Download the App 46 Review from Google Play.
Deprotonation to form an ethanol. Write the mechanism of the following reaction. CN - ion is an ambident nucleophile and can attack through both C and N.
Some Commercially Important Alcohols. Answer Sulphuric acid acts as a catalyst allowing the reaction to speed up at lower temperature. Nucleophilic attack of water on carbocation.
2CH3CH2OH----CH3CH2-O-CH2CH3 Reagent cong H2SO4 AT TEMP 413K. Show lone pairs and formal charges. CH H- CH -CH но.
The oxygen atom of ethanol has lone pair so it attacks on proton and get protonated so a positive charge is generate on oxygen of ethanol. Write the mechanism of the following reaction. N-BuBr KCN -----Et0H-H20----- n-Bu-CN KBr Share with your friends.
12-03-2022 This browser does not support the video element. The mechanism of the reaction involves the following three step. CN ion is an ambident nucleophile and can attack through both C and N.
The given reaction is. OH HSOHO 7 Write a mechanism that explains formation of the products shown in the following reaction. Br Br Br Br NaCl H0 Br OH o G.
Your service is one of Write A Mechanism For The Following Radical Halogenation Reaction the best I have ever tried. Retention refers to that phase of the molecule in which the molecular composition is preserved throughout the reaction whereas inversion refers to that mechanism in which the structure of the molecules is. Problem 8442 Your answer is correct.
Write a mechanism of the following reaction. Show lone pairs and formal charges. Posted by Imran Raza 3 years 1 month ago.
Write the mechanism of hydration of ethene to yield ethanol. The acid-catalyzed hydrolysis of an imine to a carbonyl compound and a primary amine 2. In this case it attacks through the C-atom.
2CH 3 -CH 2 -OH --- CH 3 -CH 2 -O-CH 2 -CH 3. The acid-catalyzed hydrolysis of an enamine to a carbonyl compound and a secondary amine b. OH HSOHO Problem 844_1 Correct Complete the mechanism for step one of this reaction.
Use curved arrows to show the movement of electrons in each step. How do these mechanisms. Draw and write cis or trans of the high priority groups in the product for full credit Br-OH water NaOH Hi CH3 Expert Solution.
Alcohols Phenols and Ethers. Hence this is the main example of Riemer-Tieman reaction. In this case it attacks through the C-atom.
NBuBr KCN undersetstackrelEtOH - H_2Olongrightarrow nBuCN. It is one step reaction and the mechanism is as shown above. The given reaction is an S N 2 reaction.
If the first step is the slowest and the entire reaction must wait. Write the mechanism for the following reactions. AWrite the mechanism of the following raction.
Write the mechanism of the following reaction. It follows S N 2 mechanism. NaHCO 6 Write a mechanism for the following reaction.
The reaction mechanism is the step-by-step process by which reactants actually become products. The stronger nucleophile CN-substitute the weaker nucleophile Br-The mechanism is as follows. C H 3 C H 2 O C H 2 C H 3.
Write the mechanism of the following reaction. HNO_ 3 and H_ 2SO_ 4 reacts together to form the electrophile overset NO_ 2 for the reaction. Nucleophilic attack of water as carbocation.
It is a nucleophilic substitution reaction. 08 May 2019 From now I will order papers from Do My Paper only. NBuBr KCN nBuCN.
Complete the mechanism for step two of this reaction. The complete reaction is take palce in three steps. Is there an error in this question or solution.
Draw the bromonium ion intermediate emphasizing the major position of positive charge. I Mechanism of hydration of ethene to ethanol Step-I.
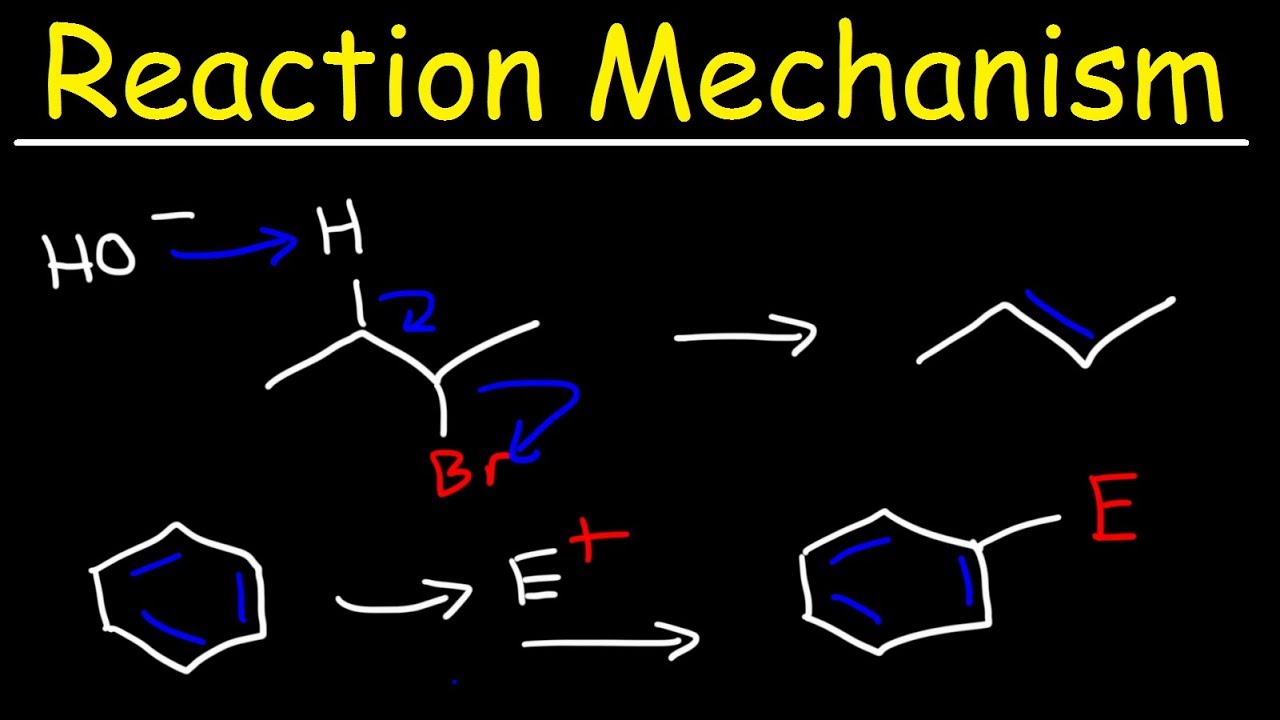
7 2 Sn2 Reaction Mechanism Energy Diagram And Stereochemistry Organic Chemistry I

Organic Chemistry Reaction Mechanisms Addition Elimination Substitution Rearrangement Youtube

Draw The Mechanism And Show The Products For The Following Sn2 Reactions Paint Program Online Painting Painting Teacher
Comments
Post a Comment